AsianScientist (Mar. 4, 2016) – A team of scientists based in Singapore has revealed the underlying mechanism for the formation and growth of a fundamental type of tissue—epithelial tubes.
The research findings open up opportunities for developing better therapies for diseases that have been linked to defects in their tubular architecture, such as atherosclerosis and polycystic kidney disease. The study was published in Nature Cell Biology.
All the major organs in the human body, such as the blood vessels, lungs and kidneys, are made up of an extensive network of tubes. These tubes—for example, arteries or intestines—function as biological pipelines that transport and deliver life-sustaining substances from one site in the body to another.
Depending on the organ in which they are formed or the specific function that they perform, the tubes vary greatly in size and shape.
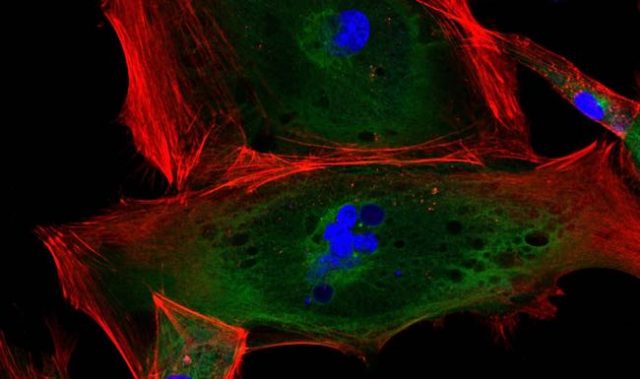
The tubes enclose hollow spaces called lumens and are primarily composed of a single or multiple layers of epithelial cells. As an important prerequisite for tube formation, epithelial cells become asymmetric or ‘polar,’ acquiring uniquely shaped ends or surfaces. However, factors that regulate the shape, size and the directional elongation of lumens into tubes remain unclear.
The present study suggests that the shape and size of some types of epithelial tubes are influenced by mechanical forces from when cells interact with their environment: the supportive extracellular matrix (ECM) that surrounds them.
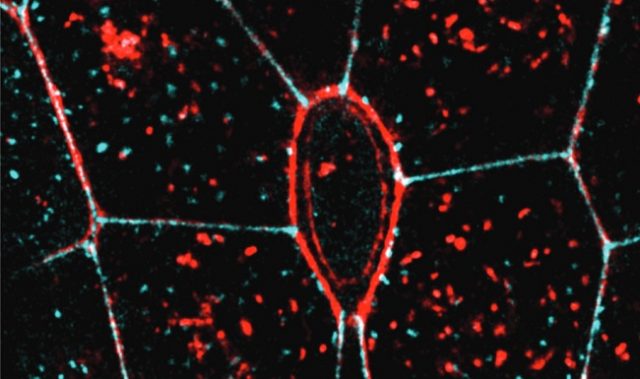
By studying the formation of bile caniculi, which are lumens formed between the contacting side surfaces of two liver cells, the scientists adopted a ‘minimal organ approach.’ This involved culturing two liver cells that can act as a functional organ unit, on artificial membranes designed with little concave impressions called microwells.
The microwells were coated with an ECM protein called fibronectin that promotes cell binding and creates growth conditions identical to that found inside cells. By coating the microwells in different patterns, the scientists altered the organization of ECM around cells. They then compared the formation of the bile caniculi and the direction of their growth.
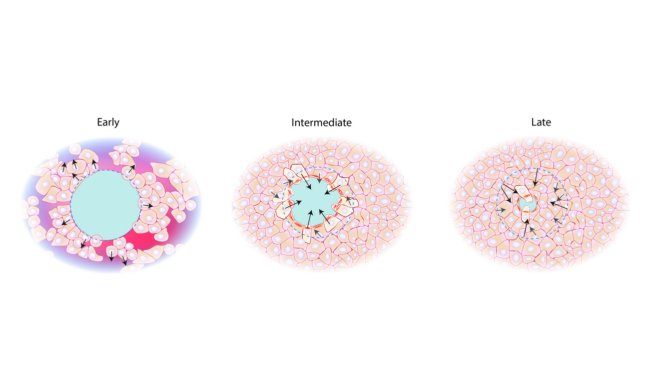
Surprisingly, they observed that lumen shape was controlled by the three-dimensional organization of ECM around cells. Furthermore, lumens showed a preference to elongate towards the free surface of the cell, away from the ECM.
According to Associate Professor Virgile Viasnoff, principal investigator at the Mechanobiology Institute (MBI) at the National University of Singapore, who led the study, “This minimal organ approach provides a unique demonstration of how biomimetic interfaces can be used to probe and understand the influence of the microenvironment on cellular processes.”
“The findings not only discovered basic principles guiding tissue morphogenesis but also shed light on the guiding principles for regenerative medicine applications,” added Professor Hanry Yu, Principal Investigator at MBI and Group Leader at the Institute of Bioengineering and Nanotechnology of A*STAR.
This study reveals, for the first time, that the interaction between cells and the ECM can control and direct the mechanical tension between cells. This mechanical tension directly influences the elongation direction of the intercellular lumen. This mechanical guidance of lumen morphology is responsible for differences in lumen shapes and sizes, formed under different microenvironmental conditions.
Viasnoff concluded that this approach offers a very promising way to understand not only tube formation, but also cell polarization. More broadly, the team expects this study to be a first step towards understanding how the environment surrounding cells affects their interactions in normal and diseased cases.
The article can be found at: Li et al. (2016) Extracellular Matrix Scaffolding Guides Lumen Elongation by Inducing Anisotropic Intercellular Mechanical Tension.
———
Source: National University of Singapore.
Disclaimer: This article does not necessarily reflect the views of AsianScientist or its staff.