AsianScientist (Nov. 29, 2016) – A team of Hokkaido University scientists has unraveled a 150-year-old mystery surrounding the surface melting of ice crystals in subzero environments by using an advanced optical microscope. Their results have been published in the Proceedings of the National Academy of Science.
Since British scientist Michael Faraday first made the observation that ice is wet on its surface 150 years ago, the question of why water on the surface of ice does not freeze in a subzero environment remained unanswered.
In their search for the underlying mechanism behind surface melting, the team used a special optical microscope jointly developed with Olympus Corp. to observe how thin water layers, or quasi-liquid layers (QLLs), are born and disappear at various temperatures and vapor pressure levels.
They found that thin water layers do not homogeneously and completely wet the surface of ice—a discovery that runs contrary to conventional wisdom. QLLs, therefore, are not able to stably exist at equilibrium, and thus vaporize.
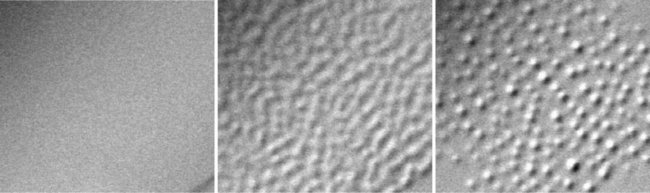
Furthermore, the team discovered that QLLs form only when the surface of ice is growing or sublimating, under supersaturated or unsaturated vapor conditions. This finding strongly suggests that QLLs are a metastable transient state formed through vapor growth and sublimation of ice, but are absent at equilibrium.
“Our results contradict the conventional understanding that supports QLL formation at equilibrium,” said Assistant Professor Ken-ichiro Murata, the study’s lead author at Hokkaido University. “However, comparing the energy states between wet surfaces and dry surfaces, it is a corollary consequence that QLLs cannot be maintained at equilibrium.”
Surface melting plays important roles in various phenomena such as the lubrication on ice, formation of an ozone hole, and generation of electricity in thunderclouds. Apart from these applications, the research could also provide a universal framework for understanding surface melting on other crystalline surfaces, the researchers say.
The article can be found at: Murata et al. (2016) Thermodynamic Origin of Surface Melting on Ice Crystals.
———
Source: Hokkaido University; Photo: Pixabay.
Disclaimer: This article does not necessarily reflect the views of AsianScientist or its staff.