AsianScientist (Jul 10, 2014) – Researchers have used gold nanoparticles to prevent biofilms from forming on medical implants. The study describing their findings has been published in the journal Applied Physics Letters.
“Implant-associated infections have become a stubborn issue that often causes surgery failure,” said Dr. Liu Xuanyong, the team’s primary investigator at the Shanghai Institute of Ceramics in the Chinese Academy of Sciences.
Designing implants that can kill bacteria while supporting bone growth, Liu said, is an efficient way to enhance in vivo osteointegration.
Titanium dioxide is able to kill bacteria itself due to its properties as a photocatalyst. When the metal is exposed to light, it becomes energetically excited by absorbing photons. This generates electron-hole pairs, turning titania into a potent electron acceptor that can destabilize cellular membrane processes by usurping their electron transport chain’s terminal acceptor. The membrane is gradually destabilized by this thievery, causing the cell to leak out until it dies.
The dark conditions inside the human body, however, limit the bacteria-killing efficacy of titanium dioxide. Gold nanoparticles, though, can continue to act as anti-bacterial terminal electron acceptors under darkness, due to a phenomenon called localized surface plasmon resonance. Surface plasmons are collective oscillations of electrons that occur at the interface between conductors and dielectrics – such as between gold and titanium dioxide. The localized electron oscillations at the nanoscale cause the gold nanoparticles to become excited and pass electrons to the titanium dioxide surface, thus allowing the particles to become electron acceptors.
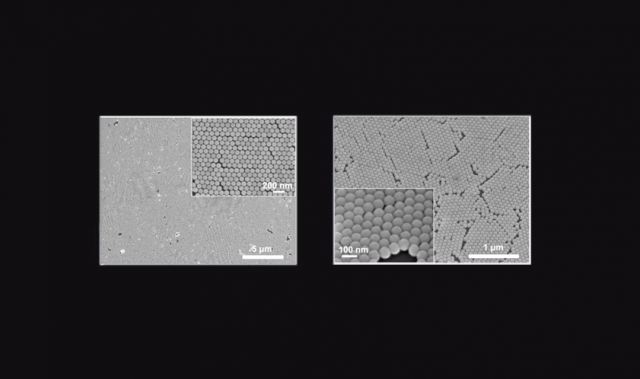
Liu and his team electrochemically anodized titanium to form titanium dioxide nanotube arrays, and then further deposited the arrays with gold nanoparticles in a process called magnetron sputtering. The researchers then allowed Staphylococcus aureus and Escherichia coli to grow separately on the arrays—both organisms were highly unsuccessful, exhibiting profuse membrane damage and cell leakage.
While silver nanoparticles have been previously explored as an antibacterial agent for in vivo transplants, they cause significant side effects such as cytotoxicity and organ damage, whereas gold is far more chemically stable, and thus more biocompatible.
“The findings may open up new insights for the better designing of noble metal nanoparticles-based antibacterial applications,” Liu said.
Further research for Liu and his colleagues includes expanding the scope of experimental bacteria used and evaluating the arrays’ in vivo efficacy in bone growth and integration.
The article can be found at: Li et al. (2014) Plasmonic Gold Nanoparticles Modified Titania Nanotubes for Antibacterial Application.
—–
Source: American Institute of Physics; Photo: Lee Haywood/Flickr/CC.
Disclaimer: This article does not necessarily reflect the views of AsianScientist or its staff.