AsianScientist (Aug. 29, 2017) – Scientists in China and the US have used optogenetic tools to precisely control the location of proteins at membrane contact sites in living cells. Their findings, published on the cover of Chemical Science, give researchers the ability to rapidly and reversibly control protein-lipid interactions in living cells.
Eukaryotic organisms, including humans, are defined by the presence of membrane bound organelles in their cells. These membranes and the cell plasma membrane are largely made out of phospholipids called phosphoinositides. Contact between different membranes is important to regulate cellular events including membrane trafficking, calcium balance and lipid metabolism.
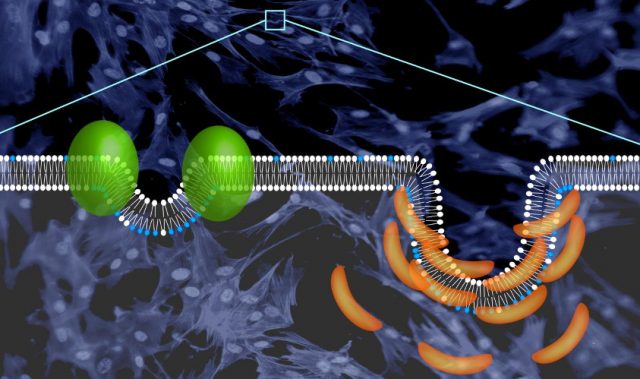
To develop a tool to control the proteins present at membrane contact sites, a joint team led by Professor Wang Junfeng at the Chinese Academy of Sciences’ High Magnetic Field Laboratory, together with Professor Huang Yun and Professor Zhou Yubin at Texas A&M University, used genetic engineering to produce a small light-responsive protein fused to phosphoinositide binding (PB) domains. The end product was a protein—OptoPB—that could rapidly and reversibly control protein translocation and thereby cause inter-membrane tethering at membrane contact sites.
Nuclear magnetic resonance analysis and molecular dynamics simulation showed that in the dark state, the PB domain docked at two negatively charged pockets within the light-responsive protein called LOV2. When LOV2 is exposed to light, it changes shape and releases the PB domain, allowing the PB domain to bind membranes. This means that OptoPB can be used as a single-component modular scaffold to deliver proteins of interest toward the plasma membrane when exposed to light.
The researchers also created a version of OptoPB that could tether to the cytosolic side of the endoplasmic reticulum (ER) membrane, OptoPBer. In the dark, OptoPBer did not bind to the membrane contact site on the plasma. However, upon blue light stimulation, OptoPBER formed a bridge between the ER and the plasma membrane by binding to the membrane contact site. Importantly, the inclusion of two to eight alpha-helical spacers in the protein structure of OptoPBer allowed the researchers to measure the distance between the ER and the plasma membrane, which was found to be in the range of 15 to 35 nanometers.
OptoPB and OptoPBer add to the optogenetic toolkit for the study of protein–lipid interactions in cells and facilitate the study of native membrane contact sites. They also allow the remote control of intermembrane communications occurring at these specialized subcellular structures.
Similar engineering strategies can be broadly extended to photo-manipulate other types of interorganellar contact sites, such as the ER-endosome-lysosome, ER-peroxisome, and ER-mitochondria membrane contact sites, thus enabling light-dependent control of cellular functions.
The article can be found at: He et al. (2017) Optical Control of Membrane Tethering and Interorganellar Communication at Nanoscales.
———
Source: Chinese Academy of Sciences; Photo: Huang Yun & Zhou Yubin.
Disclaimer: This article does not necessarily reflect the views of AsianScientist or its staff.