AsianScientist (Apr. 13, 2015) – The discovery that the superheavy element lawrencium (Lr) has an extremely low ionization potential has challenged the current arrangement of the periodic table. The study documenting these findings has been published as the cover story of Nature.
First discovered in 1961, Lr is a synthetic chemical element with an atomic number of 103. In standard periodic tables, Lr is located at the end as the last element in the actinide series, placing it as an f-block element. However, some have argued that it should be located in the d-block, having a similar chemistry to the elements scandium and yttrium.
Understanding the chemical and physical properties of Lr would allow scientists to address questions of the influence of relativistic effects and confirm its position in the periodic table. However, Lr is only accessible atom-at-a-time in syntheses at heavy-ion accelerators, and only short-lived isotopes with a half life of 27 seconds at best are known. Therefore, experimental investigations on Lr are very rare and have so far been limited to a few studies of some basic chemical properties.
In the present study, an international collaboration led by the research group of superheavy elements at the Japan Atomic Energy Agency (JAEA) have exploited a novel combination of methods and techniques to report the first and accurate measurement of the first ionization potential of Lr.
The ionization potential reflects the binding energy of the most weakly-bound valence electron in an element’s atomic shell and is strongly affected by relativity. The researchers showed that removing the outermost electron requires least energy in Lr among all actinides, 4.96eV, a figure which closely matched theoretical predictions.
For the experiment, the Institute of Nuclear Chemistry at Johannes Gutenberg University Mainz purified and prepared the exotic target material californium (atomic number 98). The material was converted into a target in Japan and then exposed to a beam of boron ions (atomic number 5).
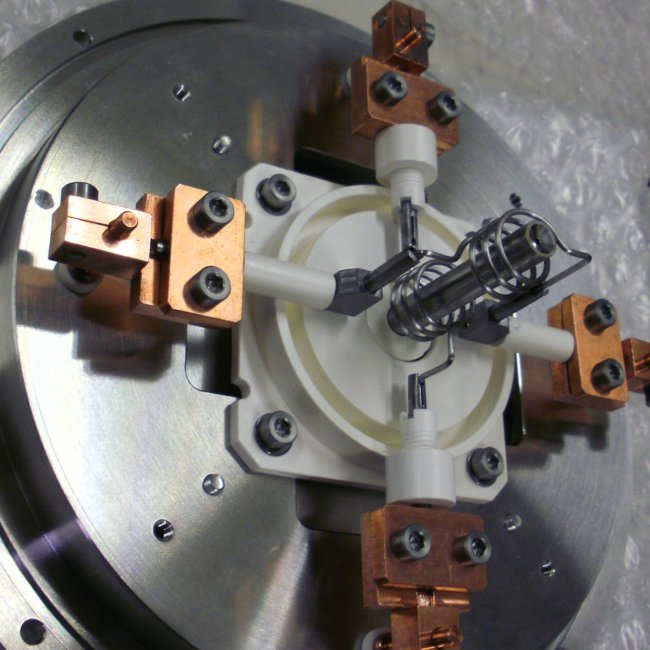
The experiment was supplemented by theoretical calculations undertaken by scientists at the Helmholtz Institute Mainz (HIM) and at Tel Aviv University of Israel using the most up-to-date quantum chemical methods to quantify the ionization energy.
The very good agreement between calculated and experimental result validates the quantum chemical calculations. The experimental technique opens up new perspectives for similar studies of yet more exotic superheavy elements.
The article can be found at: Sato et al. (2015) Measurement Of The First Ionization Potential Of Lawrencium, Element 103.
———
Source: Japan Atomic Energy Agency; Photo: Kazuaki Tsukada/JAEA.
Disclaimer: This article does not necessarily reflect the views of AsianScientist or its staff.