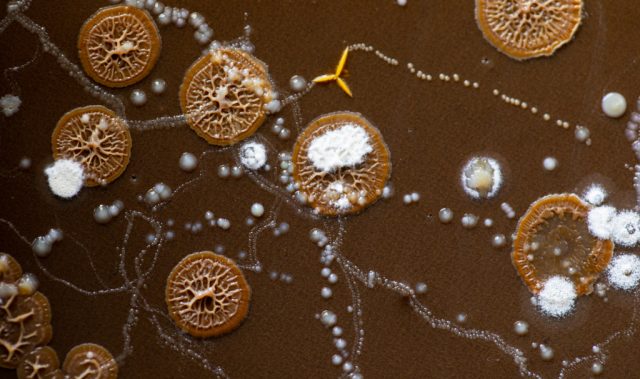
AsianScientist (Jul. 10, 2016) – Serendipity is the discovery of something valuable or pleasant without the original intent to seek it out. In the sciences, Alexander Fleming’s discovery of penicillin is arguably the most well-known anecdote of a serendipitous finding.
In a tale told many times before, Fleming left a stack of bacteria culture dishes unattended in his lab while he went for a family vacation in August 1928. Upon returning a month later, he found that one of the dishes had become contaminated with a mold. Observing that the area surrounding the mold was free of bacteria, he began to speculate that the mold was producing a substance which was lethal to the microbes. This mold was eventually identified as Penicillium notatum, and the antibacterial compound it produced came to be known as penicillin.
The Golden Age of Antibacterials
Penicillin was the first among many antibiotics that were subsequently discovered between the 1950’s and the 1960’s in a period that was called the Golden Age of Antibacterials. Approximately 50 percent of the different classes of antibiotics in clinical circulation today were discovered during this pocket in time.
For a while, humankind considered itself on the cusp of invincibility from infectious diseases, and together with better sanitation and hygiene practices across the globe, the number of deaths from bacterial infections plunged.
But to quote Michael Crichton’s Jurassic Park, “Life finds a way.”
Just as nature had handed us the means to destroy bacteria, nature had endowed the very same bacteria with the means to survive. Fast forward just five decades, and antibiotic-resistant ‘superbugs’ now hang the specter of microbial apocalypse over our heads.
One such superbug has arrived in the United States—it is resistant to colistin, an antibiotic that doctors turn to when others have failed. Rio De Janeiro’s waterways are infested with drug-resistant bacteria, at a time when the Olympic Games in Brazil is just weeks away. Multi-drug resistant tuberculosis has also surfaced in the city state of Singapore.
These recent cases, among others, really drive the message home—is this the end of the antibiotic age as we know it?
The dawn of resistance
The first recorded incidence of multi-drug resistance can be traced back to Japan in 1956, when Japanese scientist Kitamoto and colleagues identified a strain of Shigella dysenteriae, the bacteria responsible for causing severe and bloody diarrhea. They found that S. dysenteriae was resistant to four different types of antibiotics.
Three years later, two other Japanese microbiology labs demonstrated that antibiotic resistance was transferable between Shigella and other infectious gut microbes. The mechanism for transferable resistance was described in 1960 by the labs of Matsuhashi, Nakaya and Watanabe working independently but converging upon the same explanations: bacteria were trading the secrets of antibiotic resistance by means of cell-to-cell contact, sort of like a microscopic handshake.
One might expect that the world would immediately take notice of this discovery by the Japanese. In reality, however, the knowledge was slow to gain a foothold. First published in Japanese scientific journals, the language barrier meant that their discoveries flew completely under the radar of the Western world.
Even when the Western world did eventually take notice, it regarded the news with some degree of skepticism. In retrospect, this was not surprising as the Japanese work had surfaced just after the Golden Age of antibiotic discovery at a time when optimism was soaring.
Today, we are familiar with various mechanisms of antibiotic resistance. Some bacteria pump out the drug. Others build impervious shells called spores. Then there are those that ‘talk’ to each other by quorum sensing and conspire to form biofilms which completely change the dynamics of antibiotic efficacy. NEXT PAGE >>>