Joselito Sta. Ana
Senior director
Sanofi Pasteur Asia Pacific
AsianScientist (Jun. 15, 2015) – Since 2011, 15th June has been designated by the Association of Southeast Asian Nations (ASEAN) as the ASEAN Dengue Day. Sanofi Pasteur, the vaccines division of the multinational pharmaceutical company Sanofi, has supported the ASEAN Dengue Day since its inception. Sanofi Pasteur also owns the leading dengue vaccine candidate, having completed Phase III trials for their CYD-TDV vaccine.
We speak to Dr. Joselito Sta. Ana, senior director of Sanofi Pasteur Asia Pacific, on the companies plans for the roll out of the potentially game-changing vaccine and the economic impact it would have on ASEAN and beyond.
1. What is the current incidence of dengue in Asia and how much is it projected to increase over the next decade?
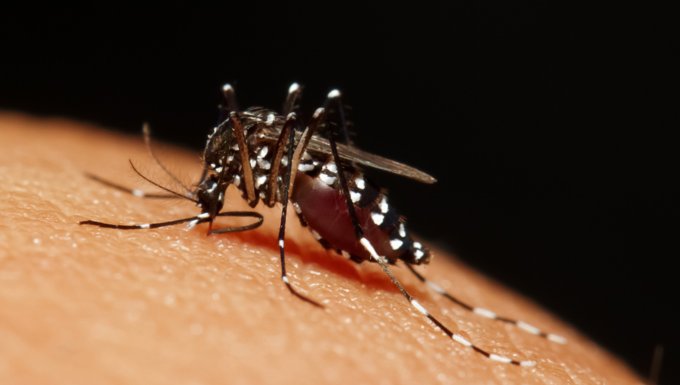
The World Health Organization (WHO) has ranked dengue as the fastest spreading mosquito-borne viral disease in the world, as seen through a 30-fold increase in global incidence over the past 50 years. Dengue has spread at an alarming speed, with a jump from only nine countries affected in 1970 to now being endemic in more than 100 nations across WHO regions.
Asia is the most impacted region representing approximately 75 percent of the global burden of dengue. It is estimated that among 2.5 billion people at risk globally, about 1.8 billion are in Asia. The current burden of disease in this part of the world averages 2.9 million dengue cases and 5,906 deaths annually across 12 Southeast Asian countries. These numbers may be understated because of unreported and undiagnosed cases and, in fact, WHO is constantly revising its dengue burden of disease citing recent improvements in reporting.
2. What are the public health and economic impacts of dengue in Asia?
In Asian countries affected by the disease, dengue remains a pervasive and year-round threat to entire populations; and exerts both a societal and public health burden and consistently ranks as a government health priority.
At a national level, the most immediate public health impact of dengue is the strain on local and national healthcare systems, which encompasses both hospitalizations and physician consultations that spike uncontrollably during outbreak situations. For instance, 70 to 80 percent of 14,000 dengue sufferers were hospitalized during the 2005 dengue outbreak in Singapore, accounting for about ten percent of hospital beds.
The economic burden of dengue extends to an international level due to its impact on travel, both from within the country, as well as from tourists visiting regions that rely heavily on this industry. A four percent decline in tourists from non-endemic countries could cause a loss of US$65 million for Malaysia and US$363 million for Thailand. This figure is particularly pressing for Thailand as tourism accounts for seven percent of its national GDP.
As governments seek to contain the disease, related healthcare costs rise accordingly. The current annual economic burden of dengue in Southeast Asia alone is estimated to be nearly US$1 billion or about US$1.65 per capita, according to a research conducted from 2001 to 2010.
3. What is Sanofi’s roll-out strategy for the CYD-TDV vaccine? When will we be able to see it on the market?
Sanofi Pasteur has completed the active phase of the two large Phase III clinical studies in Asia and Latin America with results already published in 2014. We are currently in regulatory approval process for the authority to evaluate the data comprehensively.
Sanofi Pasteur’s objective with its dengue vaccine is to have a significant impact on public health in endemic countries but the decision to use the vaccine will be made by the country themselves and not by Sanofi Pasteur.
Sanofi Pasteur’s dengue candidate vaccine will be first introduced in Latin America and Asian regions, in countries most affected by dengue. This is an entirely new access model for an innovative vaccine that could serve as a model for future prioritization of innovation solutions to meet public health needs of emerging countries.
4. What is the projected demand for the vaccine? Where do Sanofi Pasteur’s production capabilities stand at the moment?
In 2009, prior to Phase III results, Sanofi Pasteur built a dedicated production facility for the dengue vaccine in order to secure adequate supply of quality product in a timely manner to countries upon approval. This facility will have a production capacity of 100 million doses per year from 2016, this means one billion doses in ten years.
We are ready to supply the vaccine. Sanofi Pasteur’s objective with its dengue vaccine is to have a significant impact on public health in endemic countries but the decision to use the vaccine will be made by the country themselves and not by Sanofi Pasteur.
5. How will the vaccine be priced and distributed in Asia’s developing economies?
The Sanofi Pasteur dengue vaccine candidate is currently in the regulatory approval phase and hence it would be inappropriate for the company to comment at this time. It is important to note however to see that for the first time, an innovative vaccine is introduced first in middle and low-income countries. Sanofi Pasteur’s dengue candidate vaccine will be first introduced in Latin America and Asian regions, in the countries most affected by dengue. This is an entirely new access model for an innovative vaccine.
Sanofi Pasteur will encourage countries to achieve public health benefits with broad vaccination programs. We will work together with health authorities to ensure long-term sustainable access to the vaccine to support effective implementation of vaccination at levels linked to a country’s dengue burden.
6. The CYD-TDV vaccine offers incomplete protection against serotype 2, the most prevalent serotype in Southeast Asia. Furthermore, the vaccine is also less effective in younger children, the group most severely affected by dengue. Should governments make CYD-TDV their frontline approach to dengue?
Dengue epidemiology is unpredictable and changes over time. This is why the overall vaccine efficacy is so important. The consensus by public health experts is that dengue vaccination is essential to effectively control the disease.
We have now completed two large phase III efficacy trials that show consistent efficacy and safety for our vaccine. In addition, the documented clinical profile of the vaccine candidate has the potential to provide an important public health benefit in dengue endemic countries, helping them to achieve the WHO objectives to reduce dengue mortality by 50 percent and morbidity by 25 percent by 2020.
The study shows conclusive efficacy against each of the four dengue serotypes, including serotype 2 which indeed is at the lower end compared to other three. The demonstration of efficacy against each of the four serotypes is important since they all circulate in Latin America and Asia, the parts of the world most affected by dengue. Differences in efficacy by country are likely to be explained by differences in baseline antibody levels and serotype circulation.
7. What other interventions should Southeast Asian countries invest in to prevent dengue?
The consensus by public health experts is that dengue vaccination is essential to effectively control the disease. It is also foreseen that dengue vaccination and vector control measures will cohabit for maximum efficiency. We’re confident that the vaccine will be a critical tool for governments in endemic countries in their fight against dengue and to reach WHO objectives for dengue prevention.
8. Why has it been so difficult to design a good dengue vaccine?
There are four dengue serotypes that cause this disease. Their distribution varies unpredictably between regions and over time, and serotypes can re-emerge after being absent for long periods of time. Although anyone can be infected with dengue, a variety of human host characteristics can contribute to an individual contracting dengue infection, including the nature of the immune response (pre-existing and/or induced), genetic factors, chronic comorbid illnesses, and age.
This is the first vaccine successfully developed to prevent dengue disease and Sanofi Pasteur has invested significant amount of resources in identifying a unique technology platform to develop the tetravalent dengue vaccine that contains the four clinically-relevant serotypes of the disease.
9. Could you comment on the other vaccines being developed by companies such as Takeda and Merck; what impact do they have on Sanofi’s research and marketing strategy?
We don’t comment on competitors vaccines and can only address our own clinical development and industrialization program. Sanofi Pasteur dengue vaccine is the only dengue vaccine candidate to successfully complete Phase III clinical studies.
This article is part of our feature on ASEAN Dengue Day 2015. Click here to read other articles in the series.
———-
Copyright: Asian Scientist Magazine; Photo: Joselito Sta. Ana/Sanofi Pasteur.
Disclaimer: This article does not necessarily reflect the views of AsianScientist or its staff.