AsianScientist (Feb. 2, 2015) – Chemists at the Institute of Transformative Bio-Molecules (ITbM), Nagoya University and the JST-ERATO Project have developed a new method to synthesize benzene derivatives with five or six different functional groups. Their results, published in Nature Chemistry, enable access to novel functional organic materials that could not have been reached before.
First discovered in 1825, benzene is a six-membered carbon ring with a hydrogen attached to each carbon. The six hydrogens can be replaced by different substituents, making benzene an extremely versatile building block in many materials including in pharmaceuticals, agrochemicals, plastics and organic electronic devices.
Based on Burnside’s counting theorem, the number of possible substituted benzenes (N) from n different substituents is (2n + 2n2 + 4n3 + 3n^4 + n^6)/12. For example, with ten substituents, the number of possible substitution patterns on benzene will be 86,185.
Although there are a vast number of possible substituents that could be attached to benzene, many of the functional hexaarylbenzenes (HABs) possess a symmetrical structure. This is due to lack of a general method to access multi-substituted asymmetric benzenes with complete control over the position of installation.
Driven by the high necessity to access such materials, at team led by Professor Kenichiro Itami at the Institute of Transformative Bio-Molecules (ITbM) Nagoya University has devised a unique sequential approach to synthesize penta- and hexa-substituted benzene derivatives. The results are the first example of the controlled synthesis of benzene with different arene groups at all six positions ‘at-will’, demonstrating the potential of this method to synthesize useful aromatic materials in a predictable and programmed manner.
“We had been working on the development of the programmed synthesis of multiply arylated aromatic systems for over 15 years,” says Itami. “Our ultimate goal was to solve the synthetic problem of HABs, which has been extremely difficult due to the structural diversity of benzene and the limited number of synthetic methods.”
“On a substituted thiophene, we conducted a series of metal-catalyzed coupling reactions, followed by cycloaddition to synthesize HAB,” says Itami lab members Yasutomo Segawa and Shin Suzuki. “After numerous attempts to find the right reaction conditions, we were finally able to obtain the crystal structure of a propeller-shaped, radially extended HAB with six different substituents.”
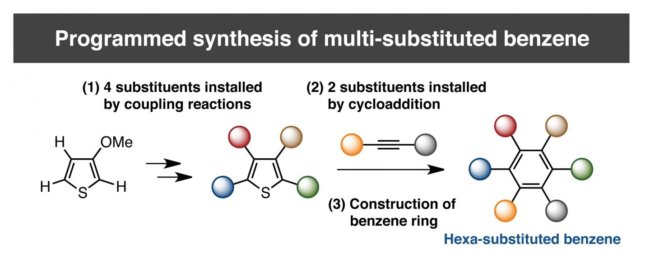
The new method has enabled the synthesis of HABs bearing five or six different substituents for the first time. Analysis of these novel unsymmetrical compounds revealed that the otherwise non-fluorescent hexaphenylbenzene could actually be made fluorescent by tuning the substituents on the exterior. These results indicate the future application of this method towards generating new molecules for molecular electronics, nanotechnology and bio-imaging.
“Programmed synthesis of HABs has long been a unresolved problem. Although the yields of our synthesis still needs to be improved, we believe that this methodology will lead to maximizing the structural diversity of benzene derivatives in a programmable fashion, which will lead to understanding structure-property relationships and help discover new functional material,” says Itami.
The article can be found at: Suzuki et al. (2015) Synthesis and Characterization of Hexaarylbenzenes with Five or Six Different Substituents Enabled by Programmed Synthesis.
—–
Source: Institute of Transformative Bio-Molecules, Nagoya University.
Disclaimer: This article does not necessarily reflect the views of AsianScientist or its staff.